The Hindu Editorial Analysis
7 October 2025
Ensuring compliance
(Source – The Hindu, International Edition – Page No. – 8)
Topic : GS 2: Issues relating to development and management of Social Sector/Services relating to Health
Context
Firms producing substandard drugs must be held accountable for their actions.
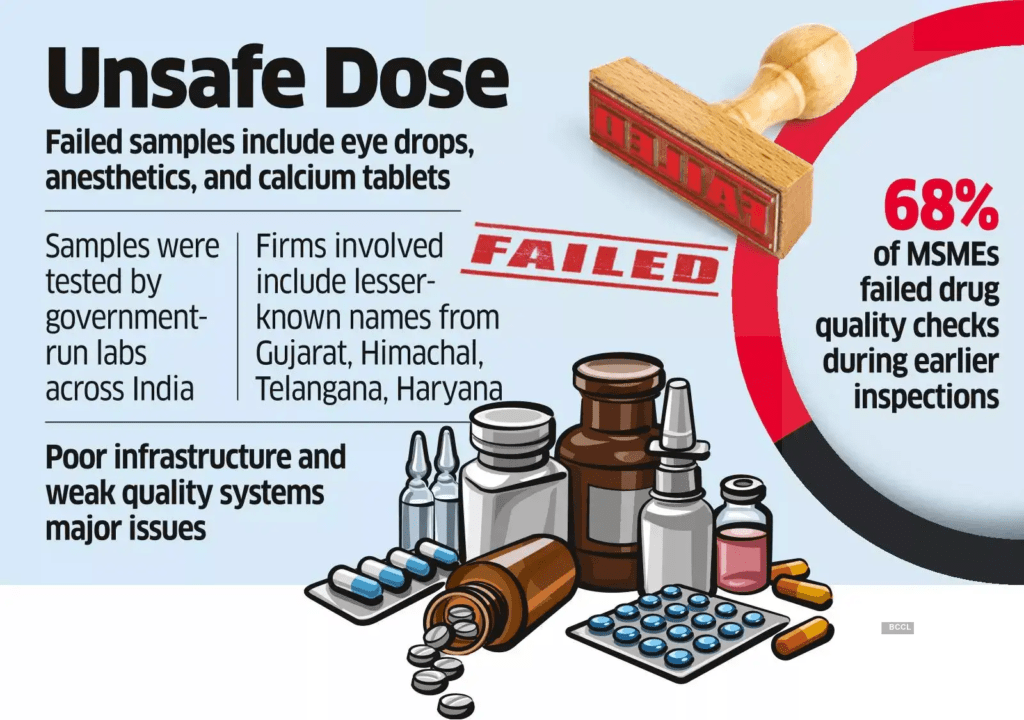
Introduction
The vision of Atmanirbhar Bharat aims to make India a self-reliant manufacturing powerhouse, but this ambition must rest on a foundation of strict quality control. Repeated lapses, particularly in the pharmaceutical sector, highlight the urgent need for stronger regulation, accountability, and ethical manufacturing practices to ensure India’s credibility as a trusted global producer.
Atmanirbhar Bharat and Quality Concerns
- Aspirational Vision: Atmanirbhar Bharat (Self-Reliant India) represents a worthy national goal, aiming to boost domestic manufacturing and global competitiveness.
- Need for Quality Assurance: However, without a strong and sustained quality control framework, this aspiration risks being undermined.
Challenges in the Pharmaceutical Sector
- Repeated Quality Issues: The pharmaceutical industry — a key pillar of India’s global image — has faced recurring concerns over drug quality, especially with cough syrups.
- Recent Controversy: The Union Health Ministry recently tightened drug compliance norms following reports of diethylene glycol (DEG) contamination in Coldrif cough syrup manufactured by a private company.
Findings and Investigations
- Triggering Incident: Tests were ordered after the syrup was suspected in the deaths of at least 16 children in Rajasthan and Madhya Pradesh.
- Contradictory Results: The Health Ministry’s early tests found no DEG in samples from these states.
- However, Tamil Nadu’s Drugs Control Department detected DEG in one batch within its jurisdiction.
- Non-Compliance Recorded: Inspections revealed several violations of Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) under the Drugs Rules.
- Source of Contamination: The contaminated batch used non-pharmacopoeial grade propylene glycol, likely introducing DEG and ethylene glycol, both nephrotoxic (kidney-damaging) substances.
- Actions Taken:
- The Central Drugs Standard Control Organisation (CDSCO) recommended cancellation of the firm’s manufacturing licence.
- A doctor who had prescribed the syrup to many of the affected children was arrested.
Need for Stronger Oversight
- Zero Tolerance Policy: India must adopt a zero-threshold approach to poor-quality drugs — no compromise on public safety.
- Proactive Enforcement: Swift monitoring and enforcement are essential; action should not wait until tragedies occur.
- Existing Framework: Robust Good Laboratory Practices already exist; the issue lies in consistent enforcement and surprise inspections.
- Accountability Measures: Every reported violation must lead to strict corrective action, setting a deterrent precedent within the industry.
- Message to Industry: The government must convey clearly that any negligence or violation endangering human life will not be tolerated.
Conclusion
Ensuring self-reliance with integrity demands unwavering commitment to drug safety and quality control. India’s growth story loses credibility if lives are endangered by negligence. A vigilant regulatory system, backed by ethical industry conduct and transparent enforcement, is essential to make Atmanirbhar Bharat not just a slogan, but a model of responsible and reliable nation-building.


